Sales

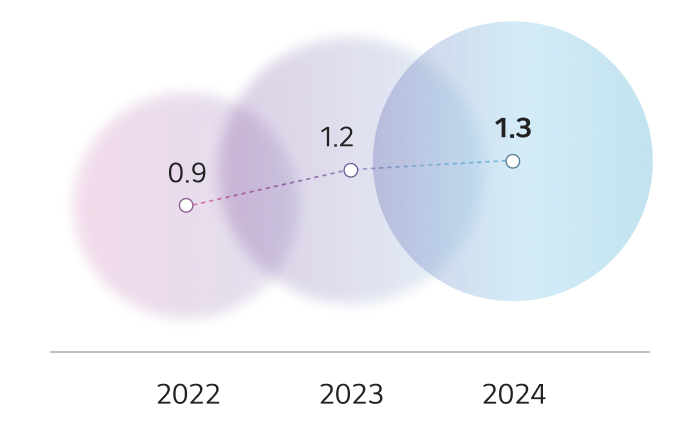
Unit : KRW trillion


Unit : KRW trillion
Toward a World-Class Innovative Drug Developer

Explore Research Preclinical Clinical
| Molecule | LR20061 |
|---|---|
| Stage | Preclinical |
| Indication | Atopic Dermatitis |
| Modality | Chemical |
| Target | Undisclosed |
| Molecule | LR19157 |
|---|---|
| Stage | Preclinical |
| Indication | Oncology |
| Modality | Chemical |
| Target | Undisclosed |
| Molecule | CUE-102 |
|---|---|
| Stage | Phase 1 (by Partner) |
| Location summary | USA |
| Indication | Oncology |
| Modality | Biologics |
| Target | WT-1 |
| Partner | Cue Biopharma |
| Molecule | Rilogrotug |
|---|---|
| Stage | Preclinical |
| Location summary | USA |
| Indication | Cachexia |
| Modality | Biologics |
| Target | anti-GDF15 IgG1 mAb |
| Molecule | AV-203 |
|---|---|
| Stage | Phase 1 |
| Location summary | USA |
| Indication | Oncology |
| Modality | Biologics |
| Target | Anti-ERBB3 IgG1 mAb |
| Molecule | LB-LR1109 |
|---|---|
| Stage | Phase 1 |
| Indication | Oncology |
| Modality | Biologics |
| Target | LILRB1 |
| Molecule | LG203003 |
|---|---|
| Stage | Phase 1 |
| Location summary | USA |
| Indication | NASH |
| Modality | NCE |
| Target | DGAT-2 |
| Molecule | Ficlatuzumab |
|---|---|
| Stage | Phase 1b/2 |
| Location summary | USA |
| Indication | Hematology |
| Modality | Biologics |
| Target | Anti-HGF/c-MET IgG1 mAb |
| Molecule | aP vaccine |
|---|---|
| Stage | Phase 2 |
| Indication | aP vaccine |
| Modality | Vaccine |
| Target | aP vaccine |
Diphtheria, Tetanus, Pertussis,Polio, Hib, HebB
| Molecule | Bivamelagon |
|---|---|
| Stage | Phase 2 |
| Location summary | USA |
| Indication | Rare Obesity |
| Modality | NCE |
| Target | MC4R |
| Partner | Rhythm Pharmaceuticals (License-out) |
| Molecule | 6 in 1 Vaccine |
|---|---|
| Stage | Phase 2 |
| Indication | 6 in 1 Vaccine |
| Modality | Vaccine |
| Target | 6 in 1 Vaccine |
Diphtheriae (D), Bordetella pertussis (wP), Clostridium tetani (T), Hepatitis B (HepB) Haemophilus influenzae type b(Hib) Vaccine-Associated Paralytic Poliomyelitis(IPV)
| Molecule | Zectivimod |
|---|---|
| Stage | Phase 2 (by Partner) |
| Location summary | China |
| Indication | Ulcerative colitis Atopic dermatitis |
| Modality | NCE |
| Target | S1P1 |
| Partner | TransThera (License-out) |
License-out, China
| Molecule | Ficlatuzumab |
|---|---|
| Stage | Phase 3 |
| Location summary | Global |
| Indication | Head and neck squamous cell carcinoma |
| Modality | Biologics |
| Target | Anti-HGF/c-MET IgG1 mAb |
| Molecule | Y-solution, MDR |
|---|---|
| Stage | NDA |
| Location summary | Europe |
| Indication | HA filler |
| Modality | HA |
| Target | HA |
| Molecule | Y-solution, China |
|---|---|
| Stage | NDA |
| Location summary | China |
| Indication | HA filler |
| Modality | HA |
| Target | HA |
